The NZSCM Guide on Theraputic Regulations
Clear guidance on navigating complex advertising rules in cosmetic medicine.
Quick Navigation
- The NZSCM Guide to the Therapuetic Advertising Regulations
- What is Advertising?
- What is Theraputic Advertising?
- What is Exempt?
- Why Does Therapeutic Advertising Have Extra Rules?
- Who Advises on the Regulations?
- Who Delivers Penalities?
- Who is the Advertising Standards Authority?
- Who are the Theraputic Advertising Prevetting Service?
- What Rules do I Need to Know?
- Brand Names
- What to Include When Naming Prescription Brands or Devices
- Approved vs Unapproved Uses of Prescription Medicines
- What is a Medical Device?
- What is the WAND Database?
- Relevant Injectable Medical Devices
- Using Before and After Images and Videos
- Good Before and After Photos
- Inadequate Before and After Photos
- Banned Imagery in Advertising
- Professional Endorsement of Prescription Medicines and Medical Devices
- Gifts, Giveaways and Discounts
- Discounts
- Comparative Advertising
- Wording of Claims
- Testimonials & Influencer Advertising: What You Can and Can’t Do
- Important Note: Your Responsibilty
- What is the Best Way I Can Stay Compliant?
- What Happens if I'm Reported for a Breach?
- What If I Need to Report an Advertising Breach?
- Resources and Contacts
The NZSCM Guide to the Therapuetic Advertising Regulations
The New Zealand government sets the regulations that govern the advertising of prescription medicines and medical devices. NZSCM doesn’t make these rules, but because they can be difficult to navigate in a cosmetic medicine context, we’ve created this guide to help clarify what’s compliant and what's not.
What is Advertising?
Any public communication controlled by you. Directly or indirectly, with the intent to influence the choice, opinion, or behaviour to whom it is addressed.
Advertising includes:
- Websites
- Television
- Radio
- Signage
- Billboard
- Social media
- Business cards
- Brochures
- Magazines
What is Theraputic Advertising?
Consumer advertising that's intended to promote:
- Clinics
- A doctor, dentist, or nurse
- Therapeutic goods, such as Botox®
- Health services
What is Exempt?
Information given during a consultation and public health information.
Why Does Therapeutic Advertising Have Extra Rules?
Because therapeutic advertising must meet a higher standard of social responsibility. When it comes to health-related treatments, there’s greater risk of misleading or influencing vulnerable consumers, so the law requires advertising to be especially truthful, balanced, and not misleading.
Who Advises on the Regulations?
- Advertising Standards Authority (ASA)
- Therapeutic Advertising Pre-vetting Service (TAPS)
- The Medical Council of New Zealand (MCNZ)
- Medicines New Zealand
Who Delivers Penalities?
- Medsafe
- The Advertising Standards Authority (ASA)
- The Medical Council of New Zealand (MCNZ)
- The Commerce Commission
Penalties can include fines up to $10,000 or six months' imprisonment.
Who is the Advertising Standards Authority?
The ASA is New Zealand’s independent, self-regulatory body for advertising. They develop and maintain the advertising codes, handle complaints, and ensure all advertising is honest, socially responsible, and not misleading.
Who are the Theraputic Advertising Prevetting Service?
Therapeutic Advertising Pre-vetting Service (TAPS) is run by the Association of New Zealand Advertisers (ANZA). It provides expert checks to make sure ads for health and cosmetic products follow New Zealand’s advertising rules before they go public. It's a helpful way to avoid misleading claims and stay compliant.
A TAPS approval number confirms your ad meets NZ advertising standards.
Your content is reviewed before it’s published.
-
Pre-publication vetting
-
Pay-per-review service
-
$140 + GST per 15 minutes
What Rules do I Need to Know?
-
Brand Names vs Generic Terms
-
Mandatory Disclaimers and Information
-
Approved vs Unapproved Indications
-
Use of Before & After Photos/Videos
-
Endorsements by Professionals
-
Gifts, Giveaways & Discounts
-
Comparative Claims
-
Language and Wording of Claims
-
Testimonials & Patient Experience Promotion
-
Use of Influencers
-
Advertising PRP (Platelet-Rich Plasma)
-
Advertising Thread Lifts
-
Advertising Exosomes
Penalties for individuals can include fines up to $20,000 or six months imprisonment.
Brand Names
If you use any brand name of a prescription medication or a medical device you must include the appropriate consumer mandatory information for that brand.
You need the mandatory statement anywhere prescription medicines and medical devices that are named or implied, including:
-
Websites
-
Social media posts (text, captions, images, videos)
-
Memes
-
Hashtags (e.g. #Botox®)
-
Reels and stories
-
Emails and digital ads
If it promotes a prescription medicine, the statement must be there. All visible brand names count!
It is expected that the use of generic terms for example, anti-wrinkle injections and dermal filler treatments, must now also have mandatory statements.
What to Include When Naming Prescription Brands or Devices
What do you need to include on each platform if you're mentioning a prescription brand or medical device:
- On websites, print, or signage: Include the full mandatory statement
- On social media: Use the short version, it has to be on the image or in the caption (not the comments)
- Font: Needs to be clear and easy to read without zooming
- Placement: Keep it close to the brand name
- Every mention matters: Include the statement on every webpage, post, or printed material where the brand is mentioned
Approved vs Unapproved Uses of Prescription Medicines
What you can advertise:
Only advertise the approved uses of prescription medicines.
For botulinum toxin products, approved aesthetic indications include:
- Forehead lines
- Crow’s feet
- Glabellar lines
Approved medical uses (commonly seen in cosmetic settings):
- Hyperhidrosis
- Chronic migraine
What you can’t advertise:
You must not mention or imply unapproved uses of botulinum toxin in your advertising, which includes websites, social media, print, and signage.
Unapproved areas (do not mention):
-
Lips/lip lines
-
Nasalis (“bunny lines”)
-
Brow lift
-
Gummy smile
-
Masseter/jawline
-
Mentalis/chin
-
Neck
-
Nose
Unapproved language (do not use):
-
Lip flip
-
Bunny lines
-
Brow lift
-
Bruxism
-
Jaw slimming
-
Dimply chin
-
Nefertiti lift
-
Haytox
Unapproved medicines:
Do not advertise or promote unapproved products like:
- PRP (Platelet Rich Plasma)
- Neuronox
These cannot be mentioned online, in advertising, or on your website.
You can discuss unapproved uses during a private consultation with a patient. Advertising rules apply to public-facing content only, not clinical discussions.
What is a Medical Device?
A medical device is any item, an instrument, apparatus, appliance, or similar, that is:
- Intended for use on or in humans for a therapeutic purpose, and
- Does not work primarily by pharmacological, immunological, or metabolic means, though these may support its function.
In other words, it helps treat or support the body without acting like a drug.
What is the WAND Database?
The Web-Assisted Notification of Devices (WAND) database is where medical devices must be registered before they can be legally supplied in New Zealand.
- Only sponsors (usually the suppliers) can access WAND; it’s not available to the public.
- If you need WAND details, ask the supplier directly.
Important: Being listed in WAND does not confirm that a product is officially recognised as a medical device.
Relevant Injectable Medical Devices
The following are classified as medical devices (not prescription medicines) and are regulated differently in New Zealand:
- Dermal fillers
- Threads (e.g., thread lifts)
- Exosomes
These still require appropriate training, regulation, and oversight, even though they are not prescription medications.
Using Before and After Images and Videos
You may only use before-and-after content in advertising if the following conditions are met:
- The patient is not identifiable
- Identifiable images are considered testimonials and are prohibited under the Medicines Act
- The images are of your own patients
- Do not use supplier or stock imagery
- No alterations or filters
- Images must not be edited or enhanced in any way (no Photoshop, smoothing apps, or filters)
- Consistent presentation
- Ensure posture, clothing, makeup, lighting, background, and camera angle are the same
- Realistic portrayal
- Avoid exaggeration or misleading visual effects
- Written informed consent
- Patients must give documented consent for their images to be used in advertising
These requirements apply to all forms of media, including social posts, websites, and printed material.
Good Before and After Photos
Good before-and-after photos should:
- Include a disclaimer like 'Individual results may vary' to reflect that outcomes differ between patients.
- Ensure the patient is not identifiable.
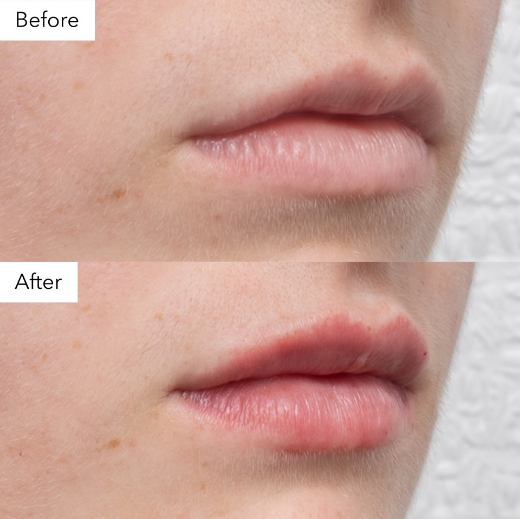
Image 1: Example of good before and after photo, cropped to the area to ensure the patient is unidentifiable.
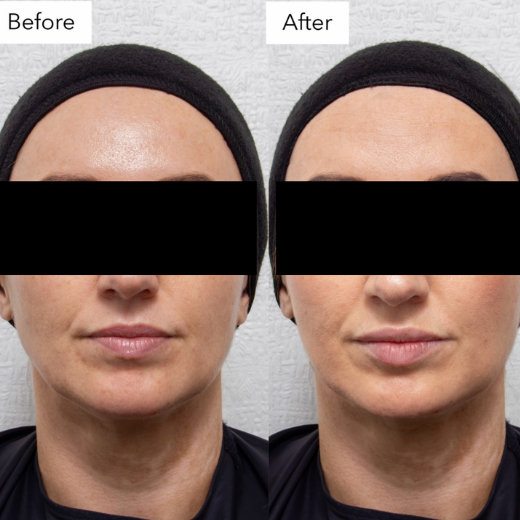
Image 2: Example of a good before and after, thick banding across the eyes and the nasal bridge to ensure the patient is unidentifiable.
Inadequate Before and After Photos
Non-compliant before-and-after photos include:
- Identifiable patients. Simply blurring the eyes, adding a thin black bar, or having the eyes closed is not enough to protect patient identity.
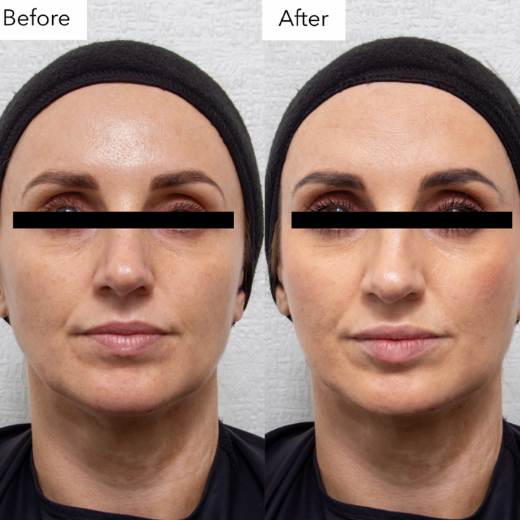
Image 3: Example of an inadequate before and after photo, the band is too narrow, and the patient is identifiable.
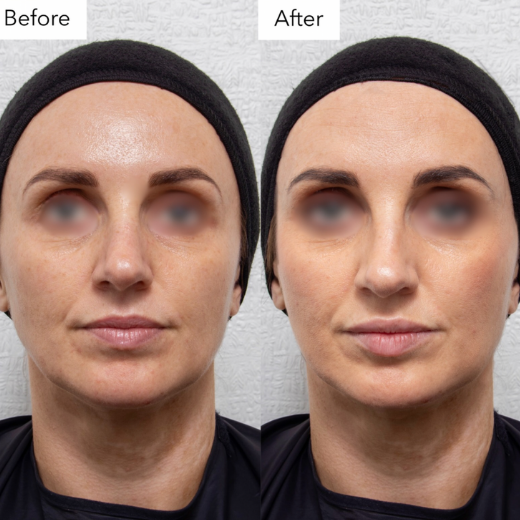
Image 4: Example of an inadequate before and after photo, the blurring does not make the patient unidentifiable.
Important: This applies to both still images and videos used in advertising.
Banned Imagery in Advertising
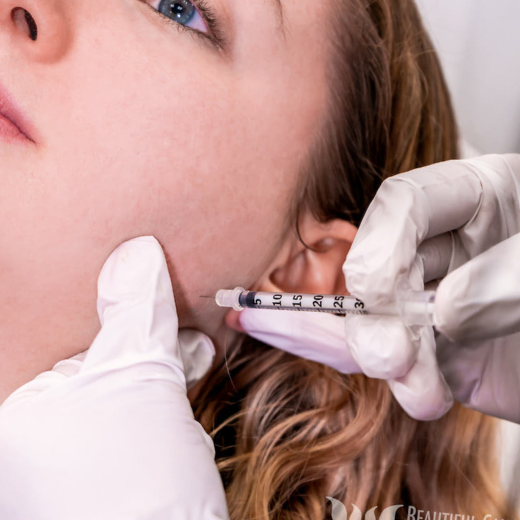 Image 5: Banned images, Unapproved Uses must not depict or suggest unapproved indications or unapproved prescription medicines.
Image 5: Banned images, Unapproved Uses must not depict or suggest unapproved indications or unapproved prescription medicines.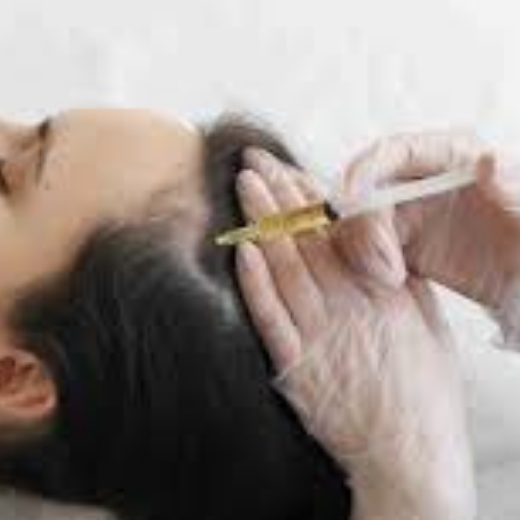
Image 5: Banned images, unapproved medication must not depict or suggest unapproved prescription medicines.

Image 6: Banned images, photoshopped mock-ups, digitally altered or AI-generated images that do not reflect real patient outcomes are not allowed.

Image 7: before and afters supplied by product companies, using supplier-provided B&A images breaches advertising rules, as they don’t show your own patients or results.
Professional Endorsement of Prescription Medicines and Medical Devices
The Medicines Act prohibits any person qualified to provide therapeutic treatment from advertising that they use, have used, or recommend the use of a prescription medicine or medical device.
This means professional endorsements are not permitted for:
-
Prescription medicines (e.g. botulinum toxin products)
-
Medical devices (e.g. dermal fillers, threads, or exosomes)
Professional endorsement includes:
-
Visible images of prescription medicines or medical devices
-
Any content where the treating practitioner is identifiable in connection with a specific product
The Medical Council of New Zealand also prohibits doctors from endorsing medical products or treatments. This includes both direct and implied promotion.

Image 8: Banned image, where prescription medicine or medical devices are visible.
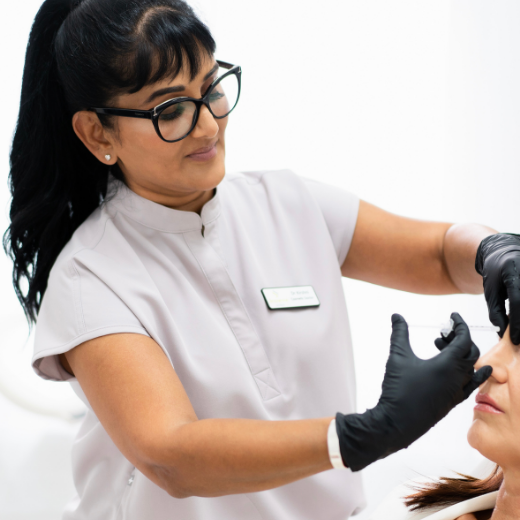
Image 9: Banned images, where the injecting clinician is identifiable.
For further detail, read the MCNZ Statement on Advertising (2022)
Gifts, Giveaways and Discounts
Gifts, giveaways, and prize draws must not include prescription medicines (e.g. botulinum toxin products) or medical devices (e.g. dermal fillers).
If your clinic offers a gift or promotion, you must clearly state that prescription medicines and medical devices are excluded from the offer.
This includes:
- Social media giveaways
- In-clinic promotions
- Loyalty or referral rewards
Advertising or promoting prescription-only products through these types of offers is not permitted under New Zealand law.
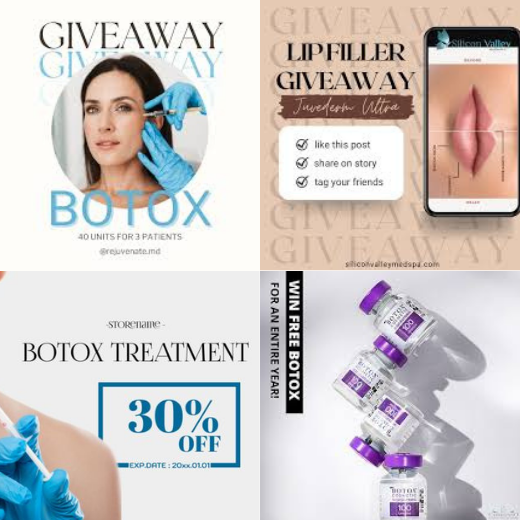
Image 10: Banned advertising, gifts, giveaways and discounts
Discounts
You can promote treatment packages as long as they do not mention a discount or imply urgency, for example, 'limited time only'.

Image 11: Compliant package advertising, it does not state a discount or imply urgency.
Comparative Advertising
Comparisons in advertising must be accurate, fair, and well-substantiated. Here’s how the rules apply in cosmetic medicine:
Prices
- Not allowed: Comparing prices only for prescription medicines or medical devices (e.g. “cheaper Botox®”)
- Allowed: Comparing the overall cost of a service that includes consultation, treatment, and follow-up—without isolating the medicine or device price

Image : Banned Advertising Not allowed: Comparing prices only for prescription medicines or medical devices.

Image : Compliant advertising, comparing the overall cost of a service that includes consultation, treatment, and follow-up without isolating the medicine or device price
Services
- You can describe your services (e.g. "advanced laser technology", "friendly, qualified staff")
- You can’t claim or imply your services are better than a competitor’s unless this can be objectively proven
- Under the Fair Trading Act, any claim must be backed by evidence, before it is made

Image :Compliant, compartive services, friendly

Image : Noncompliant, implies that are better than competitors
Products
- Comparisons between products are allowed if the evidence is robust and held on file
- Manufacturer or supplier brochures are usually not sufficient evidence
- Claims must be balanced and not misleading about the product or class of products being compared
Wording of Claims
Botulinum Toxin Products (Prescription)
- Anti-wrinkle - Claims about reducing wrinkles are permitted, as this aligns with the approved data sheets for all botulinum toxin brands. This applies to both branded and unbranded advertising.
- Anti-ageing - Claims that botulinum toxin is "anti-ageing" are not allowed. These go beyond approved indications. You can refer to reducing visible signs of ageing (like wrinkles), but you must not suggest the product itself prevents or reverses ageing.
It's acceptable to discuss the effects of ageing on the face in general terms, but avoid implying that botulinum toxin is an anti-ageing treatment.
Dermal Fillers (Medical Devices)
- All advertising claims must align with what is listed in the product’s WAND (Web Assisted Notification of Devices) notification.
- Claims not listed on the WAND cannot be made.
Note: Only product sponsors have access to the WAND database. Clinics must request this information directly from their supplier before creating advertising or promotional content.
Cosmetic Products
Do not compare a cosmetic product, device, or treatment to a prescription medicine or medical device. Doing so implies a therapeutic purpose, which is not permitted under advertising regulations.
Testimonials & Influencer Advertising: What You Can and Can’t Do
What's Not Allowed
Testimonials about any medical treatment outcomes are banned. This includes:
- Any comments about results, symptoms, or treatment effectiveness
- Statements about the doctor’s skill, treatment approach, or consultation experience
- Character references from patients, colleagues, friends, or family
You cannot:
- Solicit testimonials from patients
- Encourage patients to post on social media about their treatment
- Repost or share a patient’s treatment post
- Tag a patient in any content involving prescription medicines or medical devices
- Feature full-face images or videos of patients treated with prescription products or medical devices
- Show identifiable staff receiving injectable treatments (this is considered professional endorsement)
What is Allowed
Patients can share posts or reviews that only refer to:
- The friendliness of staff
- How they were treated at the clinic (not the treatment itself)
- The environment or non-clinical aspects of their experience
These must not mention any medical treatment, provider, or results.
Reviews
- Facebook reviews must be turned off. They are considered testimonials under your control
- Google reviews cannot be turned off and are outside your control these are allowed
- Patients must never be asked to leave a review
Influencer Advertising
A social media post is considered an ad if:
- The clinic has any input into the content
- The intent is to promote the clinic
- The influencer receives payment, free products, or free services
You cannot:
- Use influencers for injectable treatments or medical devices
- Let an influencer post about receiving a treatment from your clinic
- Share or repost their content if it includes mention of a treatment or shows you treating them
If any form of compensation is provided, the post must clearly be marked as an ad.
An Overview of Testimonials
No testimonials about treatment results, even indirect ones
- No influencers for injectables or medical devices
- No sharing or reposting patient posts about their treatment
- Focus on clinic experience only, not treatments or outcomes
Important Note: Your Responsibilty
Under New Zealand’s self-regulatory advertising model, you and your clinic are legally responsible for all content on your website and social media pages including comments or tags from others.
If a comment or post breaches the Medicines Act, the ASA Therapeutic and Health Advertising Code, or the Fair Trading Act, you must remove it. If you don’t, you may be held liable.
By everyone staying compliant, we’re able to keep the privilege of advertising in cosmetic medicine, something most countries have lost. Following the rules means we can all continue to share our work safely, professionally, and legally.
What is the Best Way I Can Stay Compliant?
Navigating the regulations can be tricky especially in cosmetic medicine.
One of the easiest and most cost-effective ways to stay compliant is to get your advertising checked by TAPS (Therapeutic Advertising Pre-vetting System).
They can help you create templates you can reuse, which keeps the process simple and affordable.
Talk to TAPS about how they can support you.
What Happens if I'm Reported for a Breach?
If you’re reported for a breach of advertising rules:
- ASA (Advertising Standards Authority) will contact you directly to discuss the complaint.
- Medsafe may send you a formal letter that requires a timely and respectful response.
Important to know:
- All ASA complaints are published on their website, regardless of the outcome. (The complainant’s name is usually not shared.)
- Medsafe complaints are not made public unless a prosecution takes place in which case, it may enter the public domain via the court system.
What If I Need to Report an Advertising Breach?
If you believe a clinic or practitioner is breaching therapeutic advertising regulations:
- For any practitioner, contact the Advertising Standards Authority (ASA) or Medsafe.
- For NZSCM-accredited doctors, or a nurse working under one of our accredited doctors, you can complete this form below to report an advertising breach. While NZSCM doesn’t enforce advertising regulations, we take concerns seriously and can follow up directly with our members where appropriate.
Important: NZSCM cannot act on concerns involving clinics or practitioners who are not members, and we do not regulate nurses independently of their supervising doctor.
Resources and Contacts
TAPS Webinars: Advertising in Cosmetic Medicine
Medical Council of New Zealand
Medsafe: Ministry of Health
Advertising Standards Authority (ASA)
Association of NZ Advertisers (ANZA) – TAPS Service